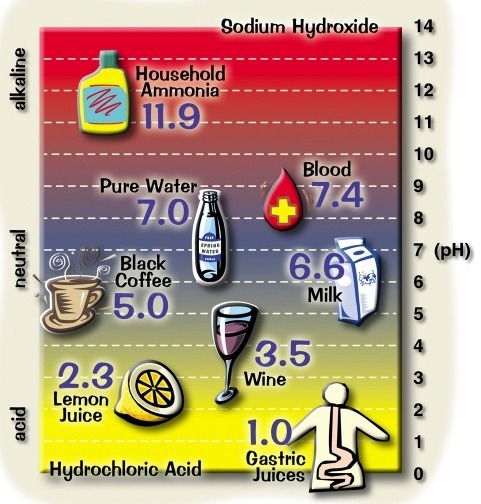
pH
The term pH is the standard measure of acidity. The maintenance of a desired pH range has important implications for life processes, agriculture, industry and the environment.
Examples of this include:
- for a person to remain healthy, the pH of blood must be maintained within a narrow range
- plant growth can be severely affected if soil pH is too high or too low, and the availability of plant nutrients and non-essential elements may be affected
- pipes and pumping equipment can be corroded in a short time if they carry water that is acidic in nature (i.e. low pH)
- plants and organisms can be adversely affected in rivers and lakes if the water pH is too high or too low.
Within the mining industry, maintenance of a desired pH range has important implications for many of the processes that take place.
The following sections describe the importance of acidity and its management for mining in Waihi, with particular emphasis on acid drainage control.
The pH scale measures hydrogen ion concentration. It indicates the acidity or alkalinity of a solution. Pure water has a pH of 7, acid solutions have a pH less than 7, and alkaline solutions a pH greater than 7.
The pH of rainwater depends upon location. Pollution and volcanic activity will affect rainwater pH locally. Acid rain is around pH 4 and can be even lower.
Like the dB scale for noise, the pH scale is logarithmic. A small change in pH represents a large change in H+ concentration. pH 1 has a hydrogen ion concentration 1,000,000 times higher than pH 7.
Buffering
Solutions that resist changes to H+ concentration are called buffers. They tend to act like sponges for H+, and prevent the concentration of added hydrogen ions from becoming too high.
Fluids in living organisms are strongly buffered. Sea water is an example found in nature. In contrast, rainwater is poorly buffered.
Buffering in water may be increased by adding alkalinity. Substances that contain the bicarbonate ion, the carbonate ion, or the hydroxide ion will increase the buffering of water.
The process of buffering is an important part of acid drainage control. Adding limestone to potentially acid forming (PAF) rock means that rainwater that contacts the rock has a higher degree of buffering. This makes it more resistant to pH changes.
Salts
When aqueous (water) solutions of an acid and a base are combined, a neutralisation reaction occurs. This reaction is characteristically very rapid and generally produces water and a salt. A salt is made up of a positively charged ion called a cation (other than H+) and a negatively charged ion called an anion (other than OH-). The negative ion is from the original acid and the positive ion is from the original base.
HCL(hydrochloric acid) + NaOH (sodium hydroxide) –> NaCl (sodium chloride or common salt) + H2O
Sodium chloride dissolved in water is a solution of salt. If the water was evaporated, the solid salt made up of cations and anions would remain as crystals.
In the mining industry, lime is often added to either neutralise or delay the onset of acidity. The neutralisation of acidity with lime may result in the development of a salt called gypsum (CaSO4). Gypsum can cause effects such as the scaling of pipelines, and these effects must be monitored and managed, and maintenance carried out where necessary.
pH and mine management
Ore processing
Lime is added to the ore prior to the addition of cyanide. This ensures that the cyanide remains in solution during the process of leaching of the precious metals. Similarly sulphuric acid is used (with superheated water) to strip the gold and silver from the carbon. At various stages throughout the processing of the ore, the solution is sampled and pH measured.
Water treatment
Lime is added to water at the water treatment plant to assist in the water treatment process. Many metals are insoluble at high pH and by adding lime, the metals flocculate and precipitate out of the water. The measurement of the pH of the water, at several points within the water treatment plant, is an important means of ensuring that the plant is performing well.
Soil acidity
In an excessively acid soil, one or more of the following effects may be observed:
- some plants simply do not grow well
- the activities of many of the soil organisms are reduced
- elements such as aluminium and manganese become so soluble they are toxic to plants
- essential nutrients such as phosphorus and molybdenum may become insoluble and unavailable to plants.
There is no such thing as a ‘correct pH’ because different plants have different pH levels at which they grow best.
Soil pH is measured in New Zealand by mixing 10 g of soil with 25 ml of distilled water.
Virtually all New Zealand soils have pH values between 4 and 8. The vast majority have pH values between 5 and 6.5, and are slightly acid.
In New Zealand agriculture, lime is generally added to raise the pH. The amount of lime that needs to be added depends upon the buffering capacity of the soil. A soil will be more buffered if it has fine texture (eg a clay loam) and/or high organic matter content, and will require more lime to raise the pH.
In Waihi, the maintenance of the soil pH within a desirable range is an important part of rehabilitation and ongoing management of the land. Lime is added as necessary when disturbed areas are rehabilitated, and in subsequent years as recommended following routine soil testing. This practice is no different to normal New Zealand agricultural pasture management.
Acid drainage
Acid formation in rock is a natural process. This can be seen over much of the Coromandel Peninsula on road cuttings in the area as iron coloured stainings on the surface of excavations and in open drainage channels. Excavation can accelerate the process of acid formation because it exposes rock to atmospheric oxygen. Groundwater, rivers and streams can potentially be adversely affected by acid drainage, and the associated increase in the solubility and leaching of metals. There are examples around the world where this has happened, and a local example exists at the old Tui mine near Te Aroha, about 60 kilometres southeast of Waihi.
Acid drainage control
A proportion of the rock in the open pit contains sulphide minerals (predominantly pyrite, FeS2). When the pyrite in these rock types is exposed to atmospheric oxygen, it can oxidise according to the following overall reaction:
FeS2 + 15/4O2 + 7/2O2 –> Fe(OH)3 + 2SO42- + 4H+
pyrite + oxygen + water –> iron hydroxide (orange precipitate) + sulphate + hydrogen ion (acid)
If this reaction is allowed to continue in an uncontrolled manner, low pH conditions can develop. Water passing through the PAF waste rock can then leach potential contaminants that could adversely affect surface water, such as rivers, streams, and groundwater.
Historically there was limited recognition of these problems in the mining industry and there are a number of particularly older mines in the world where acid drainage has resulted in major impacts on the environment. In the last 20-30 years a much better understanding of the acid drainage problem has evolved. Strategies for preventing acid drainage have been developed and are now being implemented in modern mining operations such as in Waihi.
Acid drainage control is a primary objective in the design, construction and operation of the tailings storage facilities. It is important to ensure that there are no significant adverse effects on adjacent rivers and streams.
While the waste rock at the Martha Mine open pit comprises predominantly andesite, other materials including volcanic breccia, alluvium, ash and ignimbrite are also present. The andesite and volcanic breccia rock types exhibit various degrees of weathering and are referred to as either oxidised, unoxidised or mixed.
Test work confirms that all oxidised rock, (andesite and ignimbrite) alluvium and ashes are non acid forming (NAF). In contrast, the mixed and unoxidised andesite and volcanic breccia contain variable amounts of sulphide sulphur, predominantly pyrite, with an expected range of 0.5 to 3% sulphur. Much of this rock is potentially acid forming (PAF).
Acid formation is a natural process that occurs over much of the Coromandel Peninsula. This can be seen on road cuttings in the area as iron coloured stainings on the surface of excavations and in open drainage channels. Excavation can accelerate the process because it exposes rock to atmospheric oxygen. Groundwater, rivers and streams can potentially be adversely affected by acid drainage, and the associated increase in the solubility and leaching of metals. There are examples around the world where this has happened, and a local example exists at the old Tui mine near Te Aroha, about 60 kilometres southeast of Waihi.
PAF rock must be identified and managed in such a way as to avoid significant environmental effects, both in the short term and in the long term. Quantities of PAF and NAF rock need to be determined, and considered in mine planning.
At Waihi, the concentrations of many of the environmentally important parameters (including heavy metals such as lead and mercury), are far lower than at many other mine sites, and do not pose a risk to human health or the environment.
Short term control of acid drainage
Crushed limestone is added to all PAF rock exposed during construction. This slows the rate of sulphide oxidation and prevents the development of acid conditions. To ensure that sufficient limestone has been added, slurry testing of the PAF rock is carried out.
Drainage systems are designed to ensure that stormwater runoff and drainage from PAF areas is collected and treated where necessary.
At Waihi, the concentrations of many of the environmentally important parameters (including heavy metals such as lead and mercury), are far lower than at many other mine sites, and do not pose a risk to human health or the environment.
In addition to limestone application, short term acid drainage control is achieved by limiting the area of exposed PAF material, compacting the material to reduce the infiltration of water, and covering with intermediate sealing layers.
Long term control of acid drainage
In the long term, acid drainage control is achieved by covering the waste rock with a cap that maintains a high degree of saturation, and effectively excludes oxygen.
Maintenance
Acid waters have the potential to affect pump and pipeline selection and maintenance around the site. The formation of a salt (gypsum) has the potential to affect pump and pipeline performance through scaling, and this process requires management.